Take a look around you. What’s the smallest thing you can see?
Now imagine something a thousand times smaller—so tiny that millions of these things could fit on the period at the end of this sentence. The things you’re imagining are nanoparticles, invisible to the naked eye yet powerful enough to cure currently incurable viruses like HIV.
Nanoparticle Engineering: A Brief Overview
Nanoparticle engineering focuses on the study and development of nanoparticles, which are tiny materials that are 1-100 nanometers, or a billionth of a meter. Scientists are discovering that nanoparticles can bond to targeted cells, which release drugs and block viruses from interacting with healthy cells. One of the many diseases that nanoparticles can target is HIV.
What is HIV?
HIV stands for human immunodeficiency virus, which causes HIV infection. Generally, the HIV virus attacks and destroys CD4+ T cells, which are white blood cells that fight infections in the immune system.
The main protein on the surface of the HIV virus is the gp120 protein, which attaches to a specific receptor (CD4) on a host cell, facilitating HIV transmission to healthy cells in the body. HIV viruses also contain three viral enzymes to assist the virus in replicating itself and hijacking cells.
Silver in Nanotech: How Can It Treat HIV?
Just over a century ago, surgeon B.C. Crede used silver to sterilize wounds because of its antibacterial and anti-viral properties. Today, silver nanoparticles are being produced to retain these characteristics in smaller forms to treat HIV.
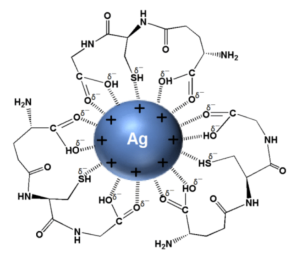
These silver nanoparticles, called AgNPs, contain a variety of components to help them function. The main component is the silver core, with its surface containing silver cations (Ag+), which are positively charged silver ions that influence the nanoparticle’s overall charge.
The ions’ positive charge allows them to attach to many biological molecules with a net negative charge, such as cell membranes, proteins, and DNA. Another vital aspect of an AgNP is its capping layer. Nanoparticles have the tendency to aggregate, or stick together and form larger particles, which reduces their effectiveness. To prevent this from happening, capping agents (such as the ones surrounding the silver particle in the diagram) are used to control the shape and size of the nanoparticle.
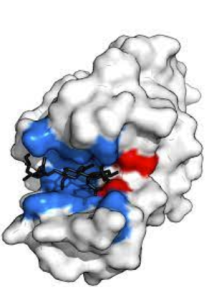
Consider the analogy of a lock and key. AgNPs, the keys, are engineered and shaped to successfully fit into HIV’s gp120 protein, or the lock. This attachment to the protein blocks the virus from binding to the the receptors of host cells, therefore preventing infection. Since HIV viruses have three viral enzymes that help them function, all of which contain an active site where they can bind to other cells, AgNPs additionally prevent further replication of the disease.
The image to the left shows the active site in blue. Because the silver ions around the core are positively charged, they can bind to the negatively charged active sites of the proteins and block the interaction between the HIV virus and the healthy cell. This physical blockage prevents the virus from hijacking the host cell, ultimately stopping the spread of infection.
The safety of the nanoparticles hasn’t been extensively researched, and the behavior of the particles may not be predictable with the research scientists have conducted so far. With the billions of possible particle interactions that go on in the body, it’s understandable why silver nanoparticles haven’t been accepted as a “cure” for HIV just yet. But with the research scientists have right now and the pace they’re moving at, the solution to one of the most notorious immune disorders may be just around the corner.