Remember watching The Magic School Bus and following Ms. Frizzle’s class on their journeys to cure medical maladies? In The Magic School Bus, Season 1, Episode 3, Ms. Frizzle and her class travel in a shrunken magic school bus through Ralphie’s bloodstream to cure his cough-inducing bacterial infection.
Well, reality isn’t that far removed from the fantastical adventures of Ms. Frizzle’s class! Much like her bus, tiny robots called nanorobots can travel in your bloodstream to cure various diseases, including certain types of cancer.
In 1959, Richard Feynman, now dubbed by most as the father of nanotechnology, gave a talk called “There’s Plenty of Room at the Bottom” to a group of physicists at Caltech’s American Physical Society. He described a theoretical process in which researchers would be able to manipulate atoms or molecules through the use of nanoscale machines. But that process isn’t just theoretical anymore. It’s been made a reality through nanorobots.
An introduction to this emerging technology.
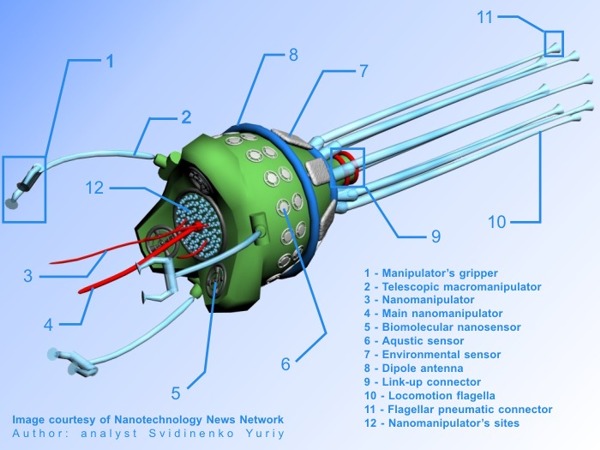
A nanorobot is a very small, fully autonomous machine made up of molecules programmed to perform a specific task. They can range from 1 to 100 nanometers in size. To put that into perspective, a sheet of paper is about 100,000 nanometers thick. Some components of a nanorobot include the power supply, motors for movement, and onboard computers. Other substructures include the payload (what the nanorobot is delivering, i.e. drug doses), a micro-camera, lasers to burn harmful material, electrodes that kill cancer cells by generating an electric current, and a swimming tail as a means of propulsion.
One very important sensor on the nanorobot chemically detects target molecules. Nanorobots are also equipped with a form of artificial intelligence called “swarm intelligence,” inspired by the behavior of social animals such as ants, bees, and termites, which collaborate on tasks without a centralized authority. These robots are the forefront of a brilliant new area in the biomedical field that is growing and evolving exponentially. In fact, the industry is estimated to reach a global net worth of over $33.63 billion by 2030 (Moore).
But why has nanotechnology been so successful? Their potential for becoming a new cure to cancer makes them invaluable in the field of oncology (the study of cancer); in fact, scientists have discovered a way to use nanorobots to shrink tumors. Researchers from Arizona State University, the National Center for Nanoscience, and the Chinese Academy of Sciences have conducted a successful experiment in which nanorobots were effective in curing cancer in mice (Pietrangelo). They injected human breast, melanoma, ovarian, and lung cancer cells into mice to give them tumors. Then, nanorobots were sent in.
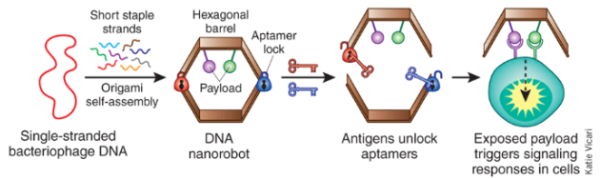
The robots contained a cage made of self-assembling single-stranded DNA, created using a technique called DNA origami. Within the cage was an enzyme called thrombin to help clot blood. On the outside of the cage were DNA aptamers, a special class of nucleic acid molecules. Inside the mice’s bloodstream, the aptamers would target a protein in a region called nucleolin, which is found in high amounts on the surface of tumor cells but not on healthy cells. Once found, the nanorobots’ cage was opened and the thrombin was able to clot the blood and stop nutrients from reaching the tumor. This cut off blood supply and quickly killed tumor tissue. This damage began within 24 hours.
Healthy tissues were completely unaffected, an impossible feat for chemotherapy or even immunotherapy. That is the number one reason why nanorobots are exalted as the holy grail of cancer prevention, because while there are many treatments for cancer, almost all of them will kill healthy cells alongside cancerous ones.
Nanorobots also helped to prevent metastasis, the secondary growth of tumors away from the initial cancer site. Afterwards, the robots were quickly cleared out (some were engineered to biodegrade, some were engulfed by white blood cells, and others were excreted) and the average survival time of the mice increased from 20 days to 45 days. However, the mice eventually still died of tumor burden, so further research is certainly needed to improve nanorobot technology.
Even if the experiment could be made fully successful, that still wouldn’t translate to widespread use in humans. As Dr. Jack Jacoub, medical oncologist, explains, “What happens in animals and humans isn’t always identical when it comes to toxicity, efficacy, and tolerability.” Experiments are still being conducted to make sure these robots would be effective in humans and only destroy cancer cells, not healthy ones.
A discussion about the benefits and drawbacks.
Current major drawbacks can be attributed to nanorobots being a relatively new technology. As they are now, developing and producing nanorobots requires far too much time and money. The process of building and synthesizing enough material to make nanorobots takes an extremely long time, making mass production unfeasible. They are also very expensive, and finding a pharmaceutical partner or large venture capital firm willing to back such a project has proven a challenge.
Even after the robots are made, the problems don’t end. The process of testing whether they work or not is lengthy as well. Scientists can’t just rely on positive results; they have to track all the movements of the robot that took place inside the body to ensure there aren’t any unforeseen side effects.
After that, it’s time to inject the robots into the bloodstream. However, based on current discoveries, nanorobots still can’t treat cancer on their own. That is because, according to neuro-oncologist Dr. Santosh Kesari, the nanorobots “won’t target single cells. A group of tumor cells [need to] come together and start making new blood vessels. Only then can the drug be delivered to those sites.”
Since nanorobots only work through blood vessels, patients would have to combine them with drug therapy, such as chemotherapy, targeted drugs, and biologic drugs, which are able target single cells.
That is not to say nanorobots are without benefit; nanotechnology for medical usage is simply an emerging field of research. A lot of research has yet to be done on the payload concept, as the purpose of nanorobots is for the carried drug dosage to be transported to the tumor location. Depending on the type and location of the targeted cancer, the features of the nanorobots’ payload will have to change for maximum efficacy.
Hearing the word chemotherapy often evokes harrowing images of hair loss and long-term hospital confinements, feelings of fear and extreme pain. Nanorobots could be the light at the end of the tunnel. Because nanorobots don’t affect healthy tissue unlike many current cancer treatments, nanotechnology for biomedicine is quickly becoming popular among the scientific community.
We are witnessing an exciting era for oncology, as well as hematology (the study of blood) and dentistry. Moving beyond the fields of medicine, nanorobots can even be used to clean up the microplastics in our oceans and reduce the cost of energy production. Futurists, such as inventor and author Ray Kurzweil, take things one step further and predict that nanorobots hold the key to age-reversing technology that will give humans eternal life (English). (Around 86% of Kurzweil’s predictions have come true so far!)
Of course, this is speculation at best. What is true is that nanorobots could become a promising new cure for cancer patients. Through time and research, they will likely transform the field of biomedical sciences forever, legions of tiny magic school buses carrying us to a healthier future.